herpes How do dormant herpes viruses (not) wake up?
by Robert Emmerich*
The herpes virus can do more than just a cold sore. For example, with herpes virus 6, more than 90 percent of all people become infected without even noticing it. But if the viruses become active again, they can cause problems – in the heart, in the nervous system … Viruses are suspected to be involved in the development of many diseases. Now researchers have discovered the mechanism of reactivation of dormant herpesvirus. This may open up new treatment options.
provider on this topic

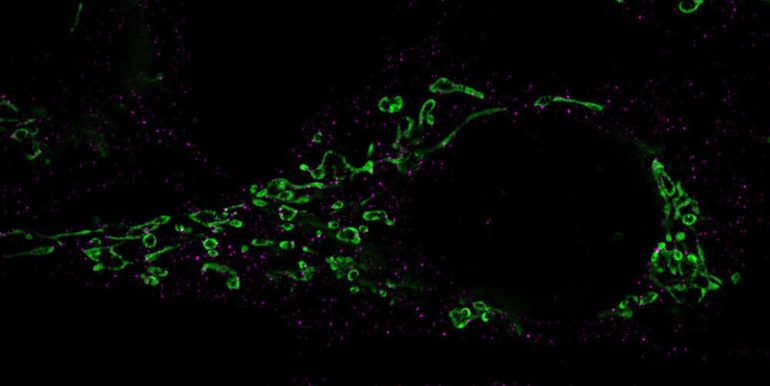
(Image: President of Virology / University of Würzburg)
Eight different herpesviruses exist in humans. All these become permanently established in the body after acute infection. Under certain circumstances, they can wake up from this resting phase, multiply and re-infect other cells. This reactivation is often associated with itchy cold sores or shingles-like symptoms.
Most herpesviruses have evolved to use small RNA molecules called microRNAs to reprogram their host cells to their advantage. A research team led by Bhupesh Prusti and Lars Dolken from the Julius-Maximilians-Universitat (JMU) Würzburg is now able to show for the first time that a viral micro-RNA acts as a master regulator to induce reactivation of the virus. Is. In the journal “Nature,” researchers present a previously unknown cellular mechanism with which human herpesvirus 6 (HHV-6) awakens from sleep.
Problems After Herpes Virus 6 Reactivation
with More than 90 percent of all people have HHV-6 Infected without even realizing it. The virus probably only causes problems when it wakes up frequently.
HHV-6 is suspected after its reactivation heart function to impress rejection of transplanted organs causes and diseases multiple sclerosis or that Chronic Fatigue Syndrome (ME/CFS) trigger. In addition, recent studies suggest that it is involved in the development of the herpes virus. a type of mental disorderThe bipolar disorder and other diseases of the nervous system may be involved.
“How herpesviruses reactivate from a dormant state is the central question of research on herpesviruses,” says JMU virologist Lars Dolken. “If we understand this, we will know how we can intervene therapeutically.” A previously unknown key to this is a viral micro-RNA called miR-aU14. That is the central switch that initiates the reactivation of HHV-6.
What does microRNA do in the cell
The regulator miR-aU14 is derived from the virus itself. Once expressed, it interferes with the metabolism of human microRNAs. In doing so, it selectively inhibited the maturation of several micro-RNAs from the miR-30 family. As a result, these important cellular micro-RNAs are no longer formed, and this in turn affects a cellular signaling pathway, the so-called miR-30/p53/Drp1 axis.
Through this pathway, viral miR-aU14 induces mitochondrial fission. These cell structures are of central importance for energy production, but also for signal transmission in defense against viruses.
Viral miR-aU14 thus inhibited the production of type I interferons—the messenger substances with which the cell reports the presence of the virus to the immune system. Because interferons are missing, the herpes virus can switch from rest to active state without manipulation. Interestingly, the Würzburg research group was also able to show that viral micro-RNA is not only essential for virus replication, but also directly triggers reactivation of the virus from the dormant state.
New therapeutic options for the treatment of herpesvirus infection
The research team now wants to understand the precise mechanism by which the viral microRNA triggers the reactivation of the virus. Furthermore, there are first indications that other herpesviruses may also be reactivated through the same mechanism. This may reveal therapeutic options to either prevent or specifically trigger reactivation of the virus by turning off reactivating cells. Another goal is to understand in detail the molecular consequences of mitochondrial fission.
This work by JMU shows for the first time that one micro-RNA can directly control the maturation process of other micro-RNAs. It also opens up new therapeutic possibilities: artificial small RNAs can be specifically designed to cleave individual members of microRNA families. Such subtle interventions were not possible before.
Original publication: Hennig T, Presti AB, Koffer BB et al. Selective inhibition of miRNA processing by a herpesvirus-encoded miRNA. Nature (2022). https://doi.org/10.1038/s41586-022-04667-4
* R. Emmerich, Julius Maximilian University of Würzburg, 97070 Würzburg
(ID:48286102)

Web guru. Amateur thinker. Unapologetic problem solver. Zombie expert. Hipster-friendly travel geek. Social mediaholic.