A team of U.S. researchers has developed copper nanowires with rich surface steps to catalyze a chemical reaction that reduces carbon dioxide (CO2) emissions while generating ethylene (C2H4), an important chemical used to produce plastics, solvents, cosmetics and other important products globally.

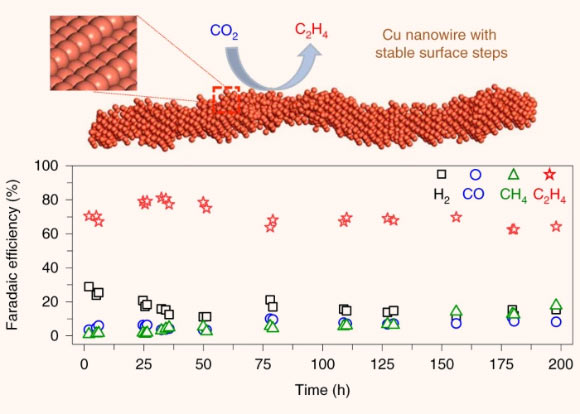
Copper represents an effective catalyst in reducing carbon dioxide to hydrocarbons or oxygenates, but it is often plagued by a low product selectivity and limited long-term stability. Choi et al report that copper nanowires with rich surface steps exhibit a remarkably high Faradaic efficiency for ethylene that can be maintained for over 200 hours. Image credit: Choi et al, doi: 10.1038/s41929-020-00504-x.
“The idea of using copper to catalyze this reaction has been around for a long time, but the key is to accelerate the rate so it is fast enough for industrial production,” said co-lead author Professor William Goddard III, a researcher in the Department of Applied Physics and Materials Science at Caltech.
“This study shows a solid path towards that mark, with the potential to transform ethylene production into a greener industry using carbon dioxide that would otherwise end up in the atmosphere.”
Using copper to kick start the carbon dioxide reduction into ethylene reaction has suffered two strikes against it.
First, the initial chemical reaction also produced hydrogen and methane — both undesirable in industrial production.
Second, previous attempts that resulted in ethylene production did not last long, with conversion efficiency tailing off as the system continued to run.
To overcome these two hurdles, Professor Goddard III and colleagues focused on the design of the copper nanowires with highly active steps — similar to a set of stairs arranged at atomic scale.
One intriguing finding of this collaborative study is that this step pattern across the nanowires’ surfaces remained stable under the reaction conditions, contrary to general belief that these high energy features would smooth out.
This is the key to both the system’s durability and selectivity in producing ethylene, instead of other end products.
The scientists demonstrated a carbon dioxide-to-ethylene conversion rate of greater than 70%, much more efficient than previous designs, which yielded at least 10% less under the same conditions.
The new system ran for 200 hours, with little change in conversion efficiency, a major advance for copper-based catalysts.
In addition, the comprehensive understanding of the structure-function relation illustrated a new perspective to design highly active and durable carbon dioxide reduction catalyst in action.
“We are at the brink of fossil fuel exhaustion, coupled with global climate change challenges,” said co-lead author Professor Yu Huang, a researcher in the Department of Materials Science and Engineering at the University of California, Los Angeles.
“Developing materials that can efficiently turn greenhouse gases into value-added fuels and chemical feedstocks is a critical step to mitigate global warming while turning away from extracting increasingly limited fossil fuels.”
“This integrated experiment and theoretical analysis presents a sustainable path towards carbon dioxide upcycling and utilization.”
The team’s paper was published in the journal Nature Catalysis.
_____
C. Choi et al. Highly active and stable stepped Cu surface for enhanced electrochemical CO2 reduction to C2H4. Nat Catal, published online September 7, 2020; doi: 10.1038/s41929-020-00504-x

Devoted web advocate. Bacon scholar. Internet lover. Passionate twitteraholic. Unable to type with boxing gloves on. Lifelong beer fanatic.